FDA Grants Pfizer's COVID-19 Vaccine Full Approval
The Food and Drug Administration on Monday gave the Pfizer-BioNTech COVID-19 vaccine its full approval ― the first to reach that status in the United States.
The FDA has resisted letting the public know precisely when to expect full approval for the coronavirus vaccines, saying in statements that it was working “as quickly as possible” and it would complete the process by January. Reports indicated that a Labor Day deadline had been set internally.
The agency has been allowing doctors and nurses to distribute the vaccine under an emergency use authorization since early December, but required more data from the manufacturer to issue full approval.
The announcement may encourage some people to get vaccinated who had been hesitant.
“While millions of people have already safely received COVID-19 vaccines, we recognize that for some, the FDA approval of a vaccine may now instill additional confidence to get vaccinated,” acting FDA Commissioner Janet Woodcock said in the agency’s statement.
The FDA said Monday that the vaccine will be marketed under the name Comirnaty for people 16 and older. As the agency continues to study the vaccine, the shots will continue to be under an emergency use authorization for people ages 12 to 15, as well as for the administration of a third dose in immunocompromised people.
Firm backing from the FDA is also expected to pave the way for vaccine mandates at organizations that have been wary of potential legal implications, although courts have so far indicated a willingness to uphold such requirements in light of a strong legal precedent to do so. A growing number of schools, health care centers, government institutions and private businesses are requiring people who enter their facilities to be vaccinated or comply with public safety measures such as testing or masking.
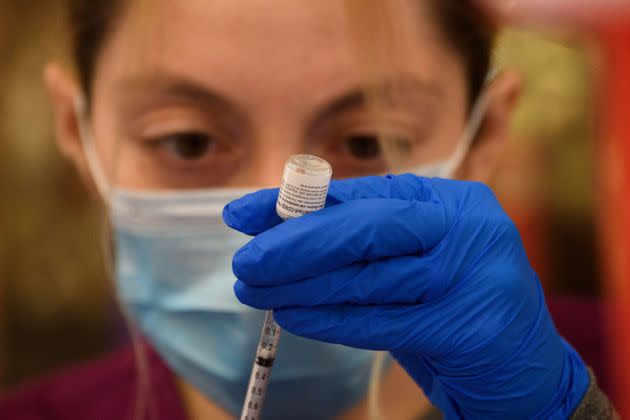
More than 200 million Pfizer shots have already been administered throughout the country, making it the most widely distributed COVID-19 drug as of Friday, according to the Centers for Disease Control and Prevention. About 143 million doses of the Moderna vaccine have been administered, followed by 14 million doses of Johnson & Johnson’s inoculation, which requires only one shot.
No vaccine is currently authorized in the U.S. for children younger than 12.
The vaccines are highly effective, but their benefits have been shown to decrease over time. Several top U.S. health officials, including Dr. Anthony Fauci, said earlier this month that Americans who received the Pfizer or Moderna vaccines should seek out a booster shot eight months after their second dose, citing new antibody data.
For the full approval of the vaccine, the FDA said it reviewed updated data from the clinical trials used to establish the emergency use authorization. The data came from approximately 20,000 vaccine and 20,000 placebo recipients ages 16 and older. More than half of the clinical trial participants were followed for safety outcomes for at least four months after the second dose, and about 12,000 recipients were followed for at least six months.
The FDA said the vaccine was 91% effective in preventing COVID-19 disease.
More than 72% of Americans have received at least one dose of a COVID-19 vaccine, but slow uptake in certain regions has allowed the virus to continue to spread, and the delta variant has made the spread easier.
Many states, largely across the South, are struggling to keep up with the rising demand for hospital beds ― and some facilities have run out of intensive care space altogether.
Some people argue that the newness of the COVID-19 vaccines is a reason not to get one. However, the shots were developed with such speed because there were little to no financial hurdles to fulfilling the FDA’s stringent safety requirements ― which called for a significant amount of data from clinical trials.
The FDA conducted the full approval for the Pfizer vaccine under its “priority review” process, which means the FDA’s goal is to take action on an application within six months instead of 10 months.
“The public and medical community can be confident that although we approved this vaccine expeditiously, it was fully in keeping with our existing high standards for vaccines in the U.S.,” Peter Marks, director of FDA’s Center for Biologics Evaluation and Research, said in a statement.
This article originally appeared on HuffPost and has been updated.

 Yahoo Sport
Yahoo Sport 









































